Amyotrophic lateral sclerosis disease-related mutations disrupt the dimerization of superoxide dismutase 1 - A comparative molecular dynamics simulation study - ScienceDirect
€ 13.00 · 4.8 (774) · En stock

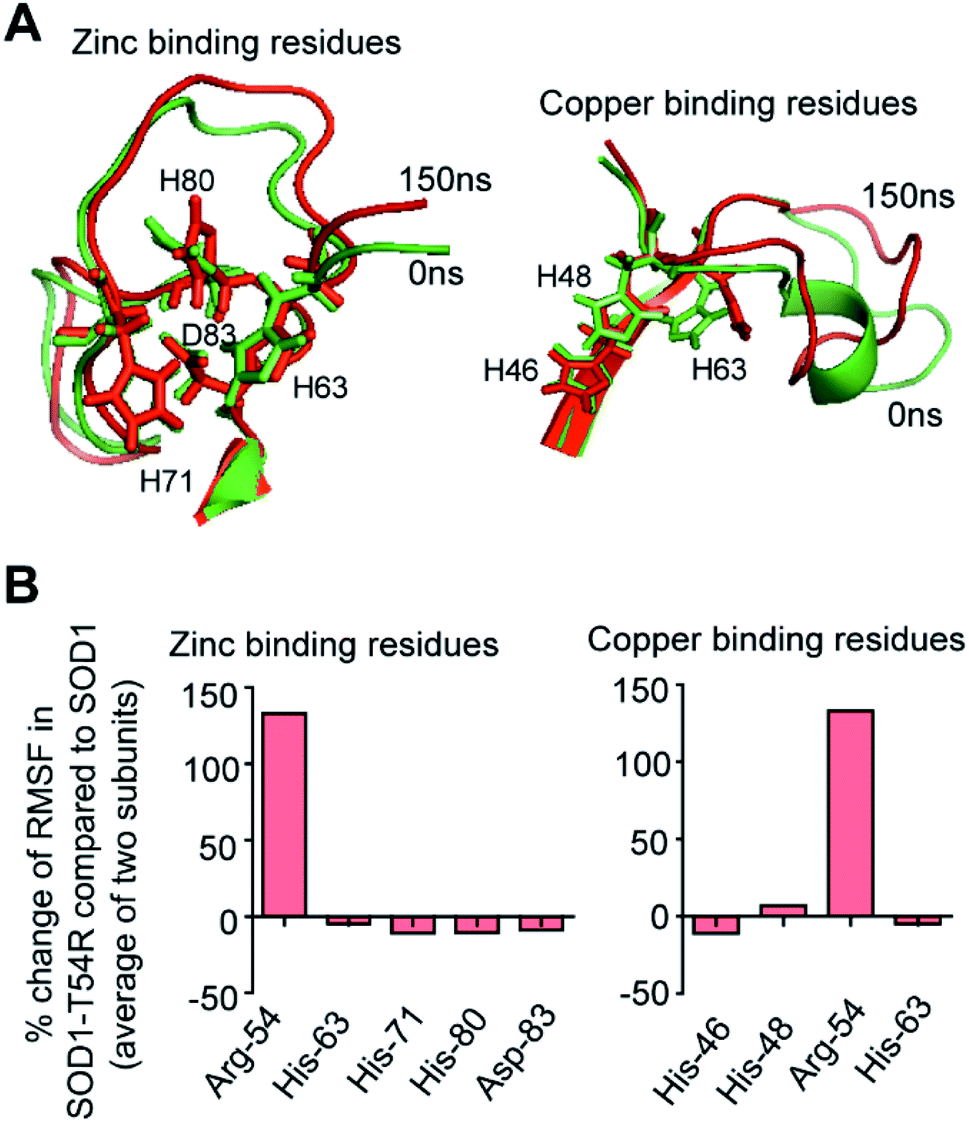
T54R mutation destabilizes the dimer of superoxide dismutase 1 T54R by inducing steric clashes at the dimer interface - RSC Advances (RSC Publishing) DOI:10.1039/C9RA09870D

Misfolding-Associated Exposure of Natively Buried Residues in Mutant SOD1 Facilitates Binding to TRAF6 - ScienceDirect
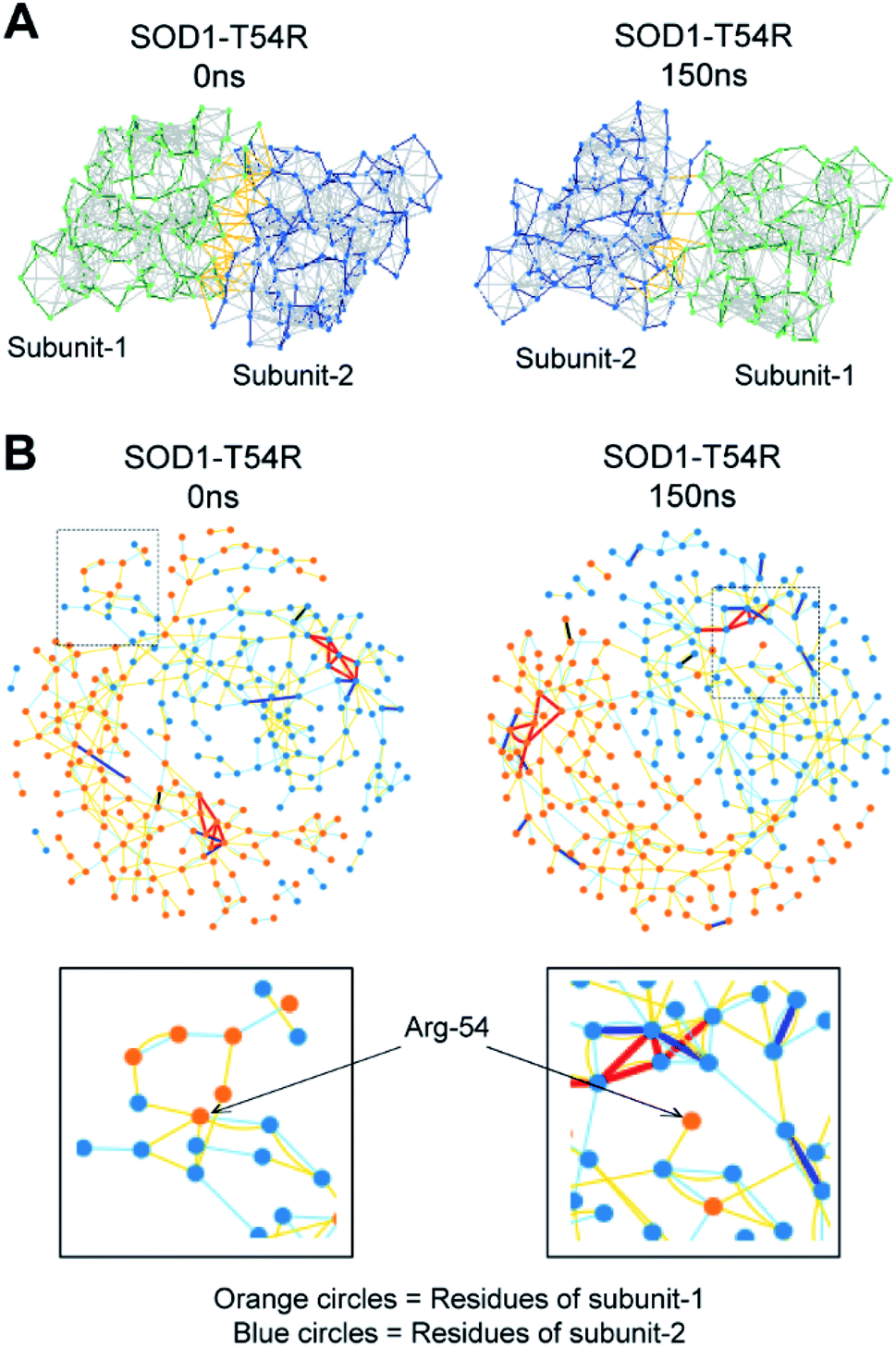
T54R mutation destabilizes the dimer of superoxide dismutase 1 T54R by inducing steric clashes at the dimer interface - RSC Advances (RSC Publishing) DOI:10.1039/C9RA09870D

Partially native intermediates mediate misfolding of SOD1 in single-molecule folding trajectories
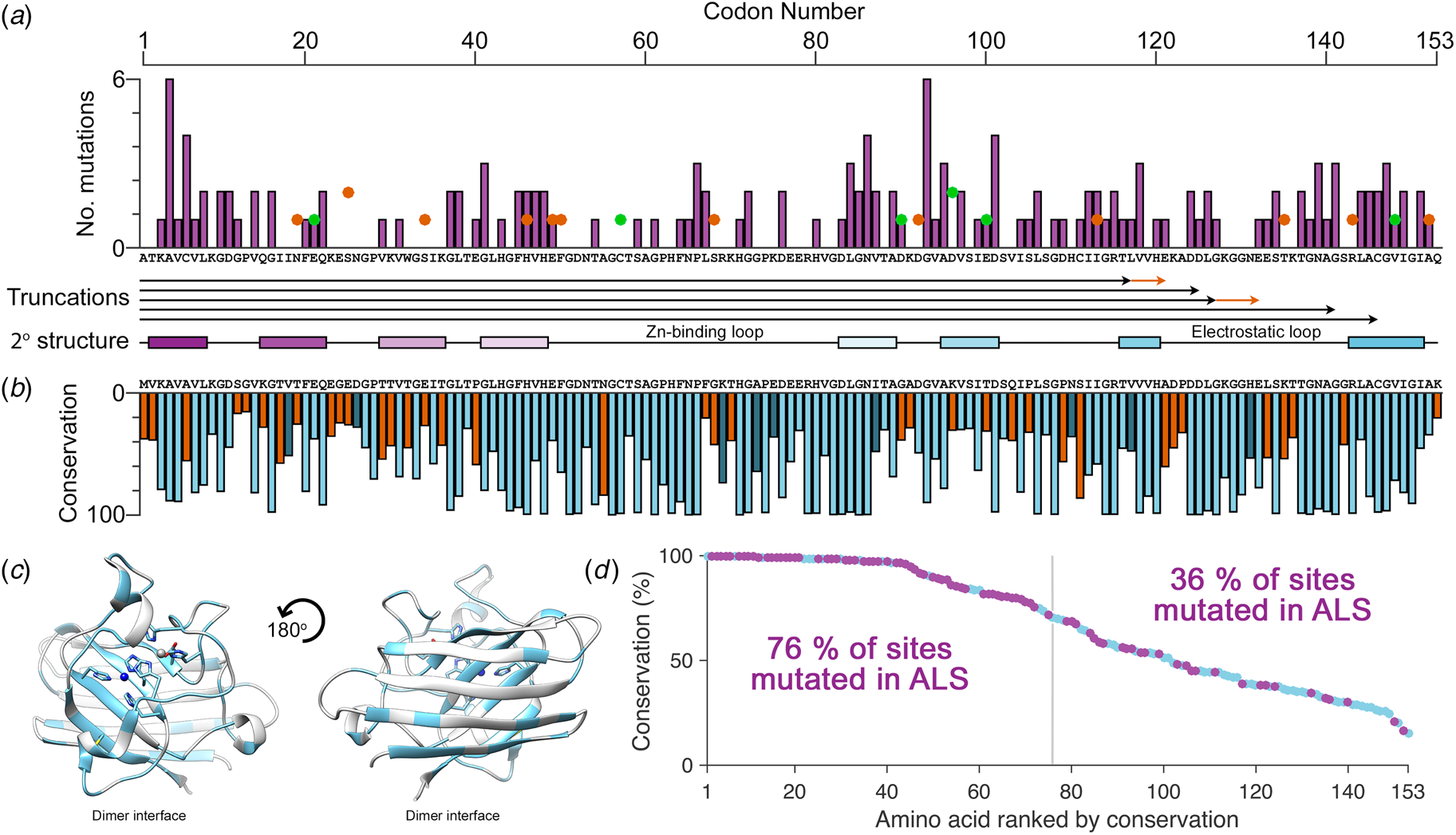
The biophysics of superoxide dismutase-1 and amyotrophic lateral sclerosis, Quarterly Reviews of Biophysics

Molecular dynamics of far positioned surface mutations of Cu/Zn SOD1 promotes altered structural stability and metal-binding site: Structural clues to the pathogenesis of amyotrophic lateral sclerosis - ScienceDirect
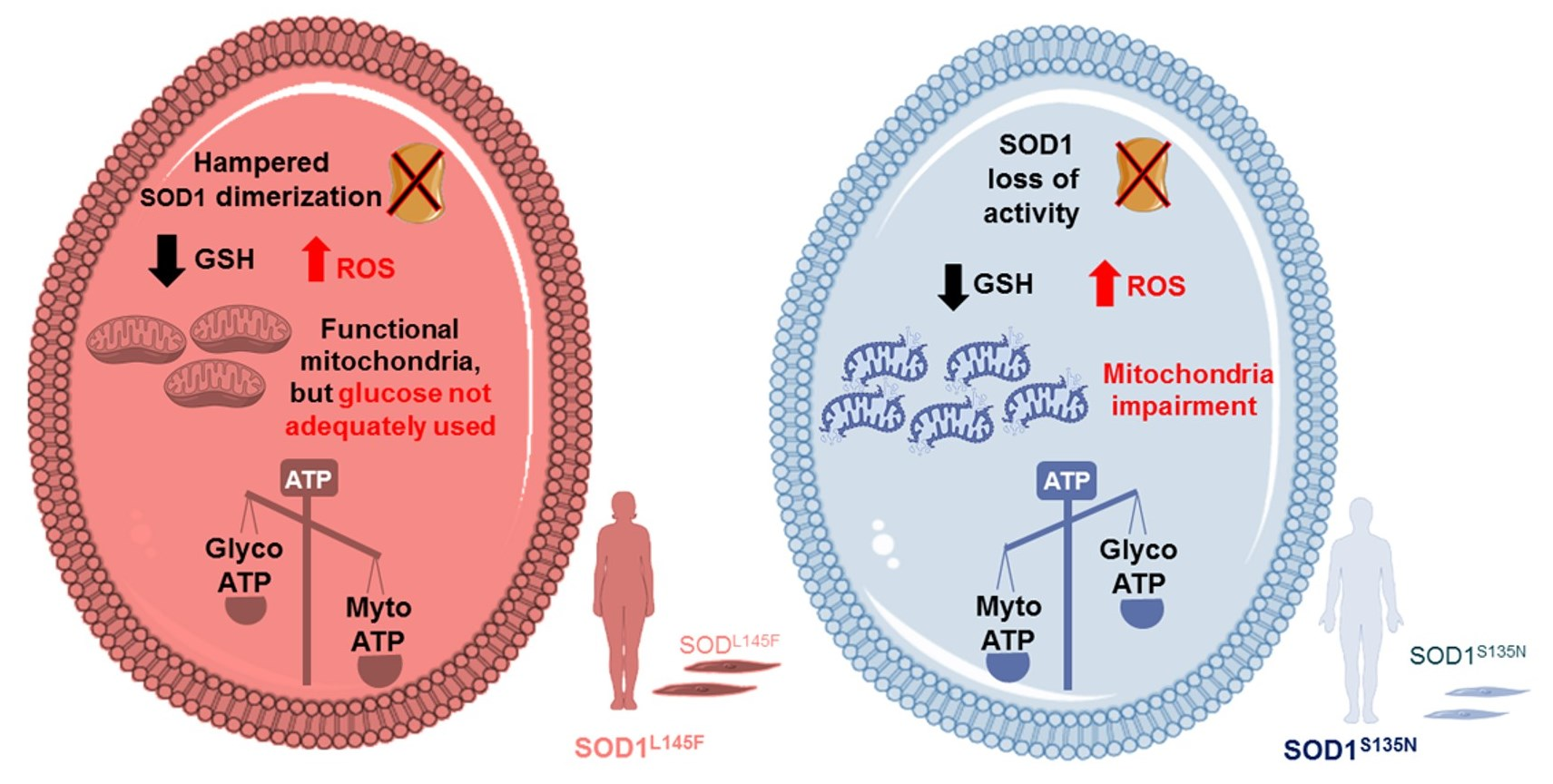
Antioxidants, Free Full-Text
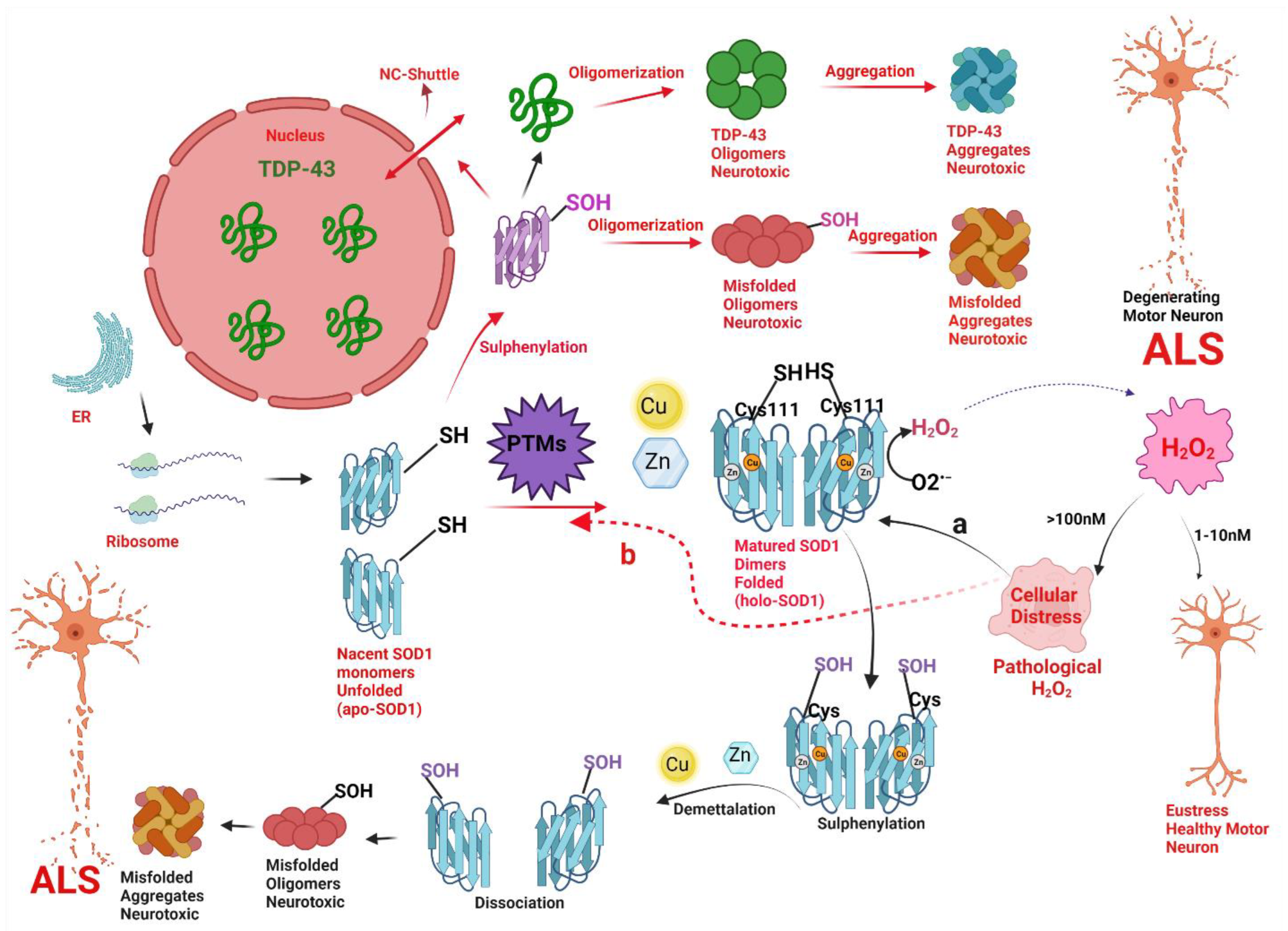
Antioxidants, Free Full-Text

Effects of Protein Crowders and Charge on the Folding of Superoxide Dismutase 1 Variants: A Computational Study









